View Natural Gas Pressure-Temperature Phase Diagram US. Furthermore, the variation law and affecting factors of the physical parameters, such as relative volume content natural gases increase with increasing temperature under constant pressure, while the density of gas decreases with increasing temperature under. High pressure may also cause a gas to change phase to a liquid.
For natural gases we are also most interested in the gas formation volume factor, bg, and the gas viscosity, μg, as these properties strongly influence gas storage (and accumulation) and gas flow.
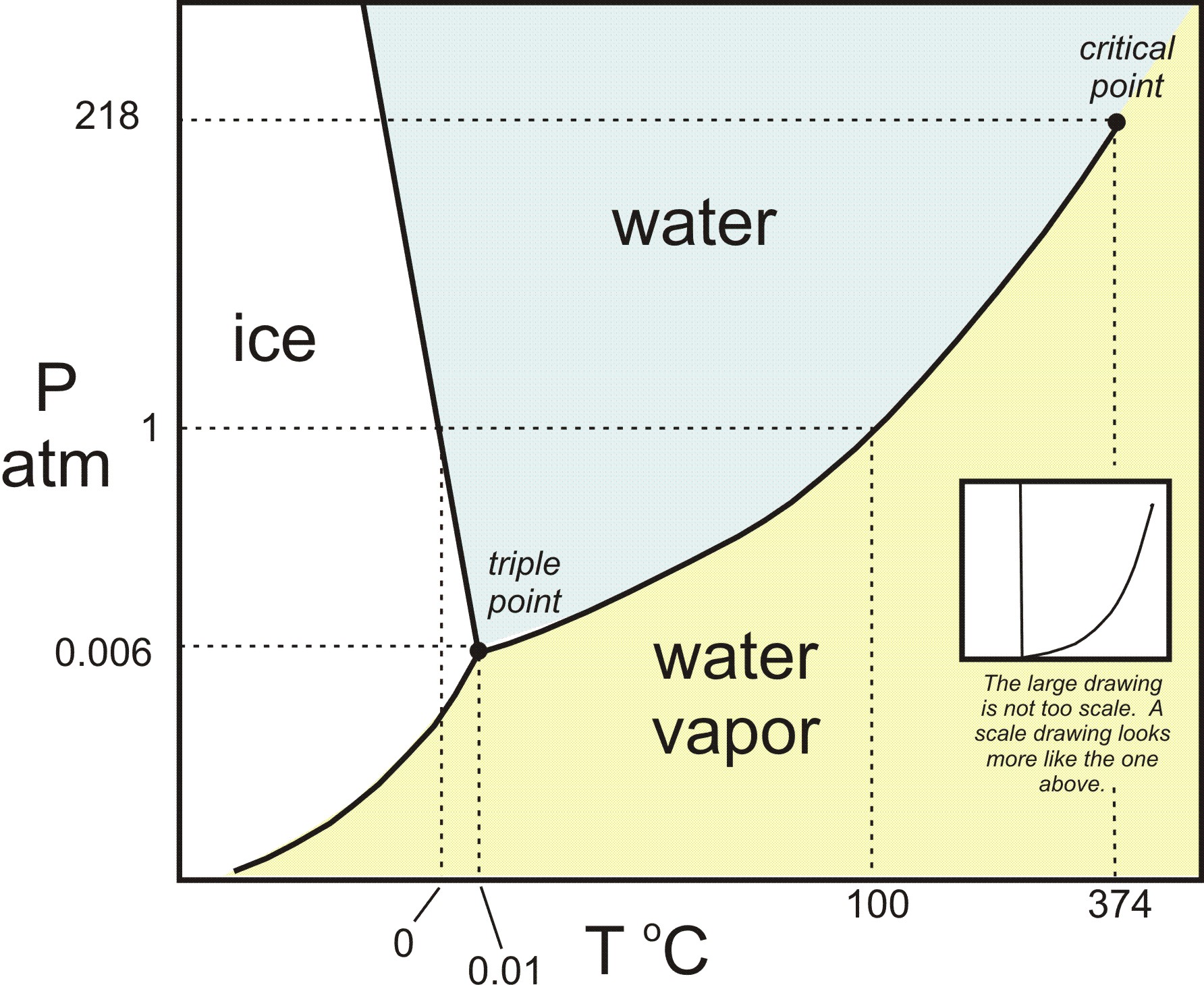
For natural gases without a liquid hydrocarbon, the p/t phase diagram is similar to that pressure/temperature diagrams for systems with inhibitors. However, at low temperature and/or high pressures the. Phase diagrams can be used in several ways. High pressure may also cause a gas to change phase to a liquid.